|
Meeting of Molecular Movie Stars
A clandestine meeting between molecules, a chemical handshake, and an exchange of energy have all been recorded on camera by scientists in the UK and Germany. Their atomic-scale footage confirms Linus Pauling's theory of chemical bonding proposed half a century ago, and could help explain molecular recognition processes important throughout supramolecular chemistry and molecular biology.
Klaus Kern, Magali Lingenfelder, and colleagues at the Max Planck Institute for Solid State Research in Stuttgart, the Fraunhofer Institute in Freiburg, and King's College London, have filmed pairs of chiral, or handed, molecules and the recognition process they undergo when they bump into each other. The study reveals the molecular body language involved as each partner changes to accommodate the size and shape of the other.
 Some molecules exist in handed, or chiral, forms—a left-handed form and a right-handed form. The building blocks of proteins, the amino acids, for instance, are chiral, as too are the proteins they form and even the genetic material, DNA, that codes for the proteins. Chiral molecules can also be made synthetically, several drugs are produced in just the left- or right-handed form for improved efficacy and to reduce side-effects. Some molecules exist in handed, or chiral, forms—a left-handed form and a right-handed form. The building blocks of proteins, the amino acids, for instance, are chiral, as too are the proteins they form and even the genetic material, DNA, that codes for the proteins. Chiral molecules can also be made synthetically, several drugs are produced in just the left- or right-handed form for improved efficacy and to reduce side-effects.
When chiral molecules attempt to meet and greet, the results depend strongly on which handed forms are interacting. Just as people can usually only shake hands if both right-hands are outstretched, so too, chiral molecules can usually only react if the appropriate handed forms meet.
Lingenfelder and colleagues used scanning tunneling microscopy to take a series of pictures that follow in detail the "encounters" of diphenylalanine molecules stuck to a substrate. Diphenylalanine is the central chemical group within fibrous molecules observed in the damaged brain tissues of sufferers of Alzheimer's disease. The "movie sequences" reveal that only molecules with the same chirality (handedness) readily aggregate into pairs and chains.
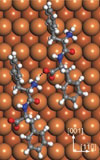 As with a handshake, it is not enough for interacting molecules to simply touch, each partner must meld their grip to make the bond firm. A close examination of the molecular movie, coupled with theoretical calculations by the team at King's College, demonstrates that molecules do adapt their shape to each other as they interact. "Our work finally demonstrates that Linus Pauling was right with his theory of intermolecular conformation of over fifty years ago," says Lingenfelder. "In molecular recognition, it is not so much the static forms that are important, but rather how well the molecules can conform to each other." As with a handshake, it is not enough for interacting molecules to simply touch, each partner must meld their grip to make the bond firm. A close examination of the molecular movie, coupled with theoretical calculations by the team at King's College, demonstrates that molecules do adapt their shape to each other as they interact. "Our work finally demonstrates that Linus Pauling was right with his theory of intermolecular conformation of over fifty years ago," says Lingenfelder. "In molecular recognition, it is not so much the static forms that are important, but rather how well the molecules can conform to each other."
InChI=1/C15H15NO2/c16-14(15(17)18)13(11-7-3-1-4-8-11)
12-9-5-2-6-10-12/h1-10,13-14H,16H2,(H,17,18)/t14-/m0/
s1/f/h17H
Angew Chem Int Edn, 2007, http://dx.doi.org/10.1002/anie.200700194
http://www.fkf.mpg.de/kern/index.html
http://www.chemspider.com/RecordView.aspx?id=4932371
|

