Down the tubes
 |
| Hicham Fenniri uses a scanning probe microscope to study structural features of a self-assembled nanotube (Purdue News Service Photo by David Umberger). |
US researchers are using the principle of molecular self-assembly to build nanoscale structures with specific dimensions and chemical properties. The resulting nanotubes could find applications in molecular wiring and other components for nanometer-sized electronic devices, new smart materials, and in novel drug-delivery systems.
Inspired by nature's own building blocks, Hicham Fenniri of Purdue University, has used the same chemical principles that make DNA strands link together to create tiny tubular structures. "The beauty of this is that, by designing the molecules that make up the system, we have perfect control over every part of the system," explains Fenniri, "We not only dictate how the molecule behaves, but we also can control the dimensions and chemical properties of the resulting nanotube." This is not so easy at the moment with the well-known carbon nanotubes, which spun out of fullerene research.
| |
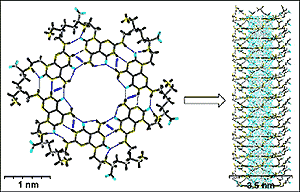 |
| Self assembly turns rosettes into tubes (adapted from JACS). Click on picture to enlarge view. |
Nanotechnology, has been a trendy buzzword for many years offering the promise of superfast computers and electrical devices and biomedical devices that could clear out arterial plaques and fix diseases, at least in the eyes of the popular science press. Fenniri's nanotubes are the first made using a self-assembly process that results in mass production and so make the possibility of nanoscale construction more feasible. "The advantage of using a self-assembly process is that it dramatically simplifies the synthetic effort," Fenniri adds, "Along with its high yields, generally close to 100%, the process also is self-correcting, so the resulting structures are predictable and error-free." The process used to make carbon nanotubes is much less reliable at present.
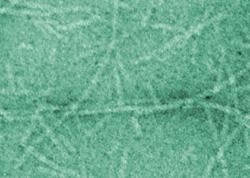 |
| Transmission electron microscopy (TEM) of the rosette nanotubes (adapted from JACS). |
Fenniri and his group used the potential pairing of cytosine and guanine seen in DNA to create a series of molecules that are "programmed" to link in groups of six to form tiny rosette-shaped rings. These rosettes then combine to form tiny, rod-like structures, or nanotubes. "The way the molecule is designed, the only way it can recognize itself is by forming a ring," he explains, "In this case, it forms a six-membered ring, or rosette. The ring is maintained by hydrogen bonds." As the molecules form a ring, the hydrophilic, water loving, ends join on the outside of the ring, burying the hydrophobic ends with an aversion for water on the inside. The rings then form stacks, naturally and spontaneously. Moreover, the hydrophilic ends on the outside of the tube have both a negative and a positive charge. As the stacks form, the ends line up with a positively charged particle on one level binding to a negatively charged particle on the next level, creating an electrostatic "belt" that wraps around the tube. This electrostatic belt serves to hold the structure together and keep it stable.
Further manipulation of these nanotubes, such as the addition of a photoactive substance to one end would create a molecular device that could pipe energy from one end to the other. The team is now studying the thermodynamics of their tubes.
Reference:
J. Am. Chem. Soc., 2001, 123, 3854-3855.*
* Articles that provide a link to a particular paper will usually take you direct to the paper, although you may need a subscription or to make a pay-per-view to the journal to access the full text. For more information on any of the publishers and how to subscribe to any journals cited in RR please go direct to the publisher's home page (http://pubs.acs.org/).

