Pure Nanotubes
Researchers in the Physics Department at Oxford University have developed a novel technique that allows them to purify carbon nanotubes and to sort those that are semiconducting from the metallic single-walled carbon nanotubes.
 | |
| Click image to magnify |
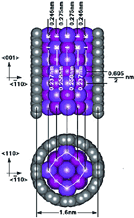
While their ball-shaped cousins, the fullerenes, may have achieved Nobel status, it is the totally tubular form of carbon - the nanotubes - that has emerged with the most interesting electronic and semiconducting properties with potential applications in optoelectronics and nanotechnology. But, as with most attempts to take a significant scientific discovery out of the laboratory and into the commercial arena, there is a serious obstacle to carbon nanotube production. Many of the potential applications are incredibly sensitive to the presence of impurities in a batch of nanotubes. Impurities, such as the ferromagnetic additives that assist in their synthesis, for instance. Moreover, metallic nanotubes in a sample of semiconducting nanotubes or vice versa, can detrimentally affect the properties of the sample.
| |  |
| Click image to magnify |
The Oxford team has developed a new method for removing the magnetic impurities without significantly affecting the yield of nanotubes. They have also proposed a method for separating the metallic from the semiconducting nanotubes using the intrinsic magnetic properties of the nanotubes themselves. This latter technique can also be used to pick out semiconducting nanotubes of different diameters. Related work to purify semiconducting nanotubes and remove graphitic contamination is also being carried out. This technique could allow researchers to experiment further with nanotubes as a new class of sub-microelectronic devices without interference from such contaminants.
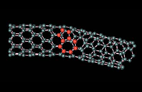
Meanwhile, Malcolm Green and Shik Tsang have patented a method for opening the ends of carbon nanotubes and filling them with a variety of other materials. The researchers use an oxidizing agent such as nitric acid to open one or both ends of at least half the nanotubes in a sample.
 | |
Louie nanotube
Click image to magnify |
Filled nanotubes often have very different properties from their hollow counterparts in terms of their magnetic and electronic behavior. Moreover, they can also be seen as potential shape-selective catalysts by analogy with the porous minerals, zeolites. An open nanotube might act as a molecular-scale reaction vessel with great size discrimination for different substrates allowing a reactant to be exposed to the contents of the nanotube in an enclosed environment. The diameter discriminating technique, also developed in Oxford, would have useful application in choosing the right nanotube for the reaction of a particular size of substrate.
The technology transfer arm of Oxford University, Isis Innovation Ltd., is now seeking companies interested in developing the commercial opportunities for this technology.
"There are currently commercial opportunities in nanoelectronics, such as those being used in transistors, also as field emission devices for display technologies," Isis physical sciences project manager, Roger Welch, told Reactive Reports. "As well as their interesting electronic properties their thermal properties make them suitable for heat dissipation in electronic components. They can also be included within plastics to make them conductive."
http://www.isis-innovation.com
http://www.chem.ox.ac.uk/researchguide/mlhgreen.html
http://en.wikipedia.org/wiki/Carbon_nanotube
|

