Critical chemistry
UK chemists have developed supercritical carbon dioxide as a clean reaction medium for the continuous formation of industrially important compounds such as ethers, acetals and ketals using solid acid catalysts.
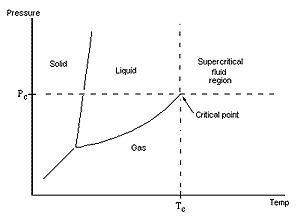 |
| Carbon dioxide goes critical schematically |
Supercritical fluids exist in a hybrid state between liquid and gas above a critical temperature and pressure and as such have some bizarre and very useful solvent properties. According to team leader Martyn Poliakoff of Nottingham University, SCFs are gases heated above their critical temperature (TC) and compressed above their critical pressure. Carbon dioxide, for instance, goes critical at close to ambient temperature, 31.3 Celsius and at 72.9 times atmospheric pressure. It is non-flammable, non-toxic, and inexpensive and in the supercritical phase has extremely low viscosity. SCFs have been used to decaffeinate coffee, clarify beer, and in natural product extraction for decades but only in the last few years have chemists begun really exploiting these properties for synthesis.
Poliakoff's team and colleagues at Thomas Swan and Co. Ltd (Consett, UK) have now developed these unusual solvents for continuous reactions in which the properties of the reaction system can be fine-tuned by small changes in the temperature and pressure so that the product profile can be optimised.
Poliakoff points out that there are far more catalytic reactions promoted by acid than any other type but, regardless of catalyst whether clay, zeolite or even graphite, they commonly suffer from rapid catalyst deactivation and a lack of selectivity. The researchers figured that supercritical carbon dioxide might make an alternative reaction medium for acid-promoted catalysis in a flow reactor because it does not degrade the catalyst to the same degree as a conventional solvent and at the same time overcome various other problems, such as product extraction and catalyst recycling.
 |
The researchers have used supercritical CO2 as medium for the conversion of alcohols to linear and branched ethers, to cyclic ethers, to aryl ethers and to acetals and ketals in good yields and, all-importantly, with high selectivities. For instance, tetrahydrofuran can readily be produced from 1,4-butanediol with 100% conversion efficiency. Once the reaction is complete the 'solvent' can be retrieved free of product, unused reactants, side products and catalyst by reducing the pressure and allowing the CO2 to boil off for collection.
The Nottingham-Swan project was initiated in 1995, partly inspired by a feature article in New Scientist magazine the previous year (NS, 1994, 6th August, Vol 143, No 1937) but details of the work under wraps until the patents process was complete.
J. Am. Chem. Soc. Vol 121, 10711*
* Articles that provide a link to a particular paper will usually take you direct to the paper, although you may need a subscription or to make a pay-per-view to the journal to access the full text. For more information on any of the publishers and how to subscribe to any journals cited in RR please go direct to the publisher's home page (pubs.acs.org).

